The global healthcare and biotechnology sectors are undergoing a major transformation with the rise of advanced therapeutic modalities such as gene therapies, cell therapies, and next-generation vaccines. At the center of these innovations lies the viral vector and plasmid DNA manufacturing industry, which plays a crucial role in enabling the development and commercialization of these breakthrough therapies. The viral vector & plasmid DNA manufacturing market has emerged as one of the fastest-growing segments within the life sciences industry, reflecting the increasing demand for reliable genetic delivery systems and biologic production platforms.
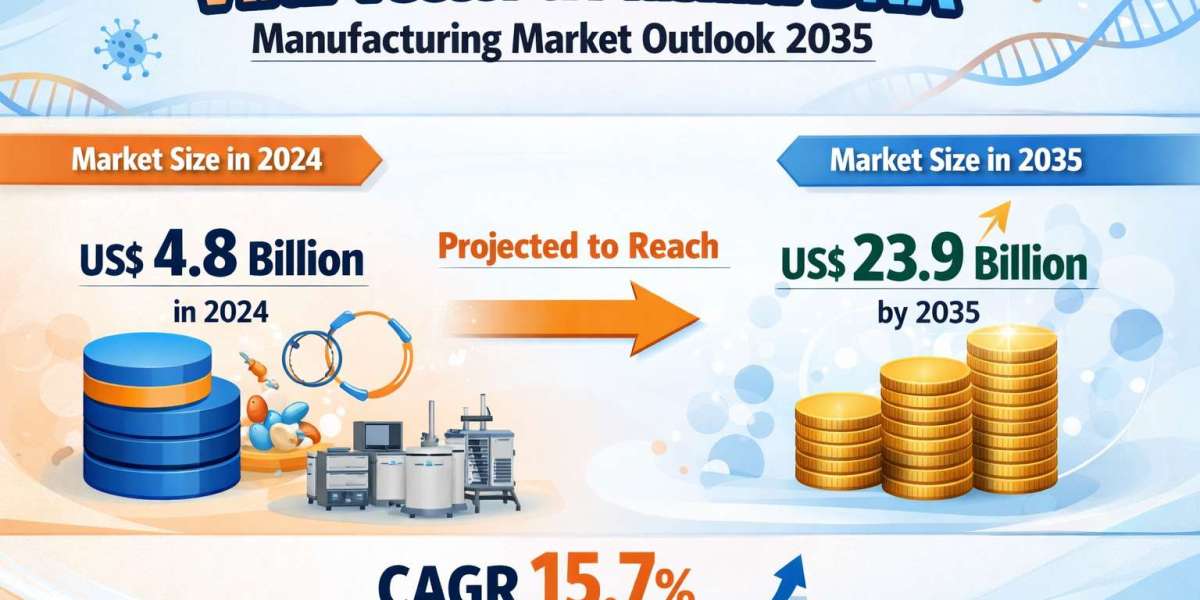
The global viral vector & plasmid DNA manufacturing market was valued at US$ 4.8 billion in 2024 and is projected to reach approximately US$ 23.9 billion by 2035, expanding at a robust compound annual growth rate (CAGR) of 15.7% between 2025 and 2035. The growth trajectory of this market is fueled by rapid advancements in gene editing technologies, increased clinical trial activity, regulatory approvals for gene-based therapies, and expanding investments by biotechnology and pharmaceutical companies worldwide.
Rising Adoption of Gene Therapy Accelerating Market Growth
Gene therapy has revolutionized the treatment landscape for several genetic, rare, and chronic diseases. Unlike traditional treatments that primarily focus on symptom management, gene therapies aim to address the root cause of diseases by modifying or replacing defective genes. Viral vectors and plasmid DNA serve as critical components in gene delivery and expression systems, making them indispensable in the production of advanced therapeutic products.
The growing gene therapy pipeline has significantly contributed to the rising demand for viral vectors and plasmid DNA. Biotechnology firms are continuously developing innovative therapies targeting complex disorders such as cancer, inherited genetic diseases, neurological disorders, and autoimmune conditions. Each new therapeutic candidate requires customized vector constructs and plasmid DNA materials, leading to increasing manufacturing complexity and higher production volumes.
Furthermore, the expansion of personalized medicine is boosting the demand for patient-specific gene therapies. Personalized therapies require flexible and scalable manufacturing systems capable of producing smaller batch sizes with high precision and regulatory compliance. This trend has encouraged manufacturers to invest heavily in advanced production technologies and modular facility designs.
Expanding Clinical Trials and Regulatory Approvals Strengthening Industry Momentum
The increasing number of clinical trials and regulatory approvals for gene and cell therapies is another major driver propelling the viral vector and plasmid DNA manufacturing market. As therapies advance from early-stage development to late-stage clinical trials and commercialization, the need for large-scale manufacturing capacity grows significantly.
Regulatory agencies across the globe are implementing stringent quality and safety standards for gene-based therapeutics. These standards require manufacturers to maintain high levels of consistency, purity, and potency in their production processes. Consequently, companies are investing in advanced automation, high-throughput analytical tools, and standardized manufacturing platforms to ensure compliance with regulatory requirements.
The surge in clinical trial activities has also encouraged the diversification of manufacturing platforms. Facilities are increasingly being designed to accommodate multiple vector types, including adeno-associated viruses (AAV), lentiviruses, adenoviruses, and retroviruses. Such versatility allows manufacturers to support various therapeutic programs simultaneously while improving operational efficiency and reducing production bottlenecks.
Technological Advancements Transforming Manufacturing Processes
Technological innovation continues to play a pivotal role in shaping the viral vector and plasmid DNA manufacturing landscape. The industry is witnessing rapid adoption of flexible manufacturing technologies such as single-use bioreactors, closed-system workflows, and perfusion-based production processes. These technologies help minimize contamination risks, reduce turnaround times, and enhance production scalability.
Advancements in downstream purification processes are also contributing to improved product quality and yield. High-capacity chromatography techniques, advanced filtration systems, and nuclease clearance technologies are being integrated into manufacturing workflows to achieve higher levels of product purity and throughput. These innovations are particularly crucial for meeting the stringent quality standards associated with gene and cell therapies.
Moreover, the integration of digital technologies such as process analytics, automation, and artificial intelligence is enabling manufacturers to optimize production efficiency and ensure consistent product quality. Real-time monitoring and predictive analytics are helping companies detect potential deviations early in the manufacturing process, thereby improving operational reliability.
Viral Vectors Dominating Market Share
Among the various product segments, viral vectors account for the largest share of the viral vector and plasmid DNA manufacturing market. In 2024, viral vectors represented approximately 62.4% of the total market share, highlighting their widespread application in gene therapy and vaccine development.
Viral vectors are highly efficient in delivering therapeutic genes into target cells with precision and long-term stability. Their ability to facilitate sustained gene expression makes them ideal for treating complex genetic disorders. Lentiviral vectors, adenoviral vectors, and adeno-associated viral vectors are widely utilized in the development of advanced therapies and vaccines.
Continuous improvements in vector design, safety profiles, and delivery efficiency are further strengthening the dominance of viral vectors in the global manufacturing landscape. Researchers and manufacturers are focusing on developing next-generation vectors with enhanced targeting capabilities and reduced immunogenicity to expand their therapeutic applications.
Plasmid DNA Serving as a Critical Manufacturing Component
Plasmid DNA plays a vital role as a foundational raw material in the production of gene therapies and DNA-based vaccines. Plasmid manufacturing involves bacterial fermentation processes, typically using Escherichia coli cultures, followed by extensive purification steps such as lysis, chromatography, and filtration.
High-quality supercoiled plasmid DNA is essential for ensuring efficient gene expression and therapeutic efficacy. The increasing complexity of gene therapy constructs has led to higher demand for GMP-compliant plasmid manufacturing processes. Manufacturers are focusing on improving plasmid stability, purity, and scalability to support the growing pipeline of gene-based therapeutics.
Growing Importance of Contract Development and Manufacturing Organizations
Contract development and manufacturing organizations (CDMOs) have become key contributors to the viral vector and plasmid DNA manufacturing market. Many biotechnology companies prefer outsourcing manufacturing operations to CDMOs due to the high capital investment required for establishing advanced manufacturing facilities.
CDMOs offer comprehensive end-to-end services, including plasmid DNA supply, vector drug-substance production, process development, and fill-finish capabilities. Strategic partnerships between therapy developers and contract manufacturers enable faster technology transfer, improved manufacturing efficiency, and reduced time-to-market for new therapies.
Flexible commercial agreements such as capacity reservation models, toll manufacturing, and risk-sharing partnerships are becoming increasingly common in the industry. These collaborations help align business incentives and ensure reliable supply chains for advanced therapeutic products.
Regional Landscape Highlighting North America’s Dominance
North America continues to lead the global viral vector and plasmid DNA manufacturing market, accounting for approximately 43.8% of total market revenue in 2024. The region’s dominance is attributed to its strong biotechnology ecosystem, advanced research infrastructure, and substantial investments in gene therapy research and development.
The presence of major biopharmaceutical companies, leading research institutions, and well-established GMP manufacturing facilities further strengthens North America’s market position. Additionally, supportive regulatory frameworks and significant funding from both government and private organizations create favorable conditions for innovation and manufacturing expansion.
Europe also represents a significant market share, driven by increasing research activities, growing clinical trials, and supportive government initiatives. Meanwhile, the Asia Pacific region is expected to witness rapid growth during the forecast period due to expanding biotechnology investments, increasing healthcare expenditure, and rising adoption of advanced therapeutic technologies.
Competitive Landscape Characterized by Strategic Expansion and Innovation
The viral vector and plasmid DNA manufacturing market is highly competitive, with several global players investing in capacity expansion, technological innovation, and strategic partnerships. Leading companies such as Thermo Fisher Scientific Inc., Lonza, Merck KGaA, Takara Bio Inc., Sanofi, WuXi Biologics, and Fujifilm Biotechnologies are actively strengthening their manufacturing capabilities to meet rising industry demand.
Market participants are focusing on establishing multi-site manufacturing facilities and modular production units to enhance geographic reach and operational flexibility. Standardized manufacturing platforms are being adopted to simplify technology transfer and reduce regulatory risks associated with product development.
Recent industry developments highlight the growing emphasis on capacity expansion and technological advancement. For instance, ProBio launched a state-of-the-art cell and gene therapy center of excellence in New Jersey in 2025, focusing on large-scale viral vector and plasmid DNA manufacturing. Similarly, ArcticZymes Technologies introduced a new GMP-grade nuclease designed to improve viral vector manufacturing efficiency.
Emerging Trends Shaping the Future of the Market
Several emerging trends are expected to shape the future of the viral vector and plasmid DNA manufacturing market. The increasing adoption of modular and flexible manufacturing technologies will enable companies to respond quickly to evolving therapeutic demands. Sustainability initiatives, including energy-efficient production methods and waste reduction strategies, are also gaining importance among manufacturers.
The growing integration of automation and workforce upskilling is expected to enhance production efficiency and ensure consistent product quality. Additionally, proactive regulatory engagement and collaborative partnerships will play a crucial role in accelerating product approvals and commercialization.
As gene therapies continue to gain regulatory acceptance and commercial success, the demand for reliable and scalable manufacturing solutions will increase significantly. This is likely to create substantial growth opportunities for both established manufacturers and emerging biotechnology companies.
Future Outlook
The viral vector and plasmid DNA manufacturing market is poised for remarkable growth over the next decade. The continuous expansion of gene therapy pipelines, technological advancements in manufacturing processes, and increasing investments in biotechnology research are expected to drive sustained market expansion.
Manufacturers are likely to focus on developing flexible, scalable, and cost-efficient production platforms to support the growing demand for personalized and advanced therapies. Collaborative partnerships, capacity expansion initiatives, and digital transformation will remain key strategic priorities for market participants.
With increasing global awareness about the potential of gene-based therapies to address unmet medical needs, the viral vector and plasmid DNA manufacturing industry is expected to play a central role in shaping the future of modern medicine.