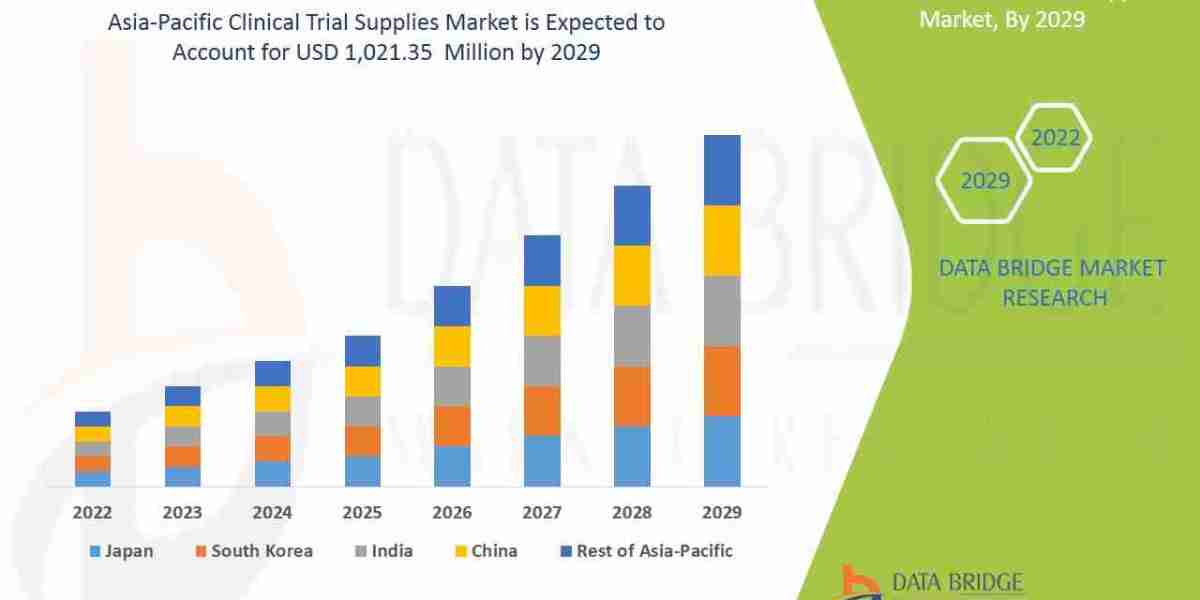
Executive Summary Asia-Pacific Clinical Trial Supplies Market
Data Bridge Market Research analyses that the market is growing with a CAGR of 9.2% in the forecast period of 2022 to 2029 and is expected to reach USD 1,021.35 million by 2029.
The market research analysis of Asia-Pacific Clinical Trial Supplies Market report considers the way people live, think, and spend so that technologies, the acquisition strategies to be employed and things required for building and upholding the brand image gets used properly. What is more, proven tools and techniques have been employed for generating market research reports which provides the creative ideas to make your product more effective and impressive in the competitive market. Asia-Pacific Clinical Trial Supplies Market is one of the most relevant, exclusive, valuable, fair and creditable international market research reports which convert complex market insights into simpler version from the end users point of view.
This Asia-Pacific Clinical Trial Supplies Market research report is created with an analysis of information and data which is collected by communicating with people. This business report helps organizations in every sphere of business to make better decisions, to answer even the toughest business questions and reduces the risk of failure. To have a powerful business growth and success in this swiftly changing marketplace, companies must plump for a broad range of information which can be accomplished through this report. That’s why global market research analysis report is very essential. Such Asia-Pacific Clinical Trial Supplies Market report ultimately leads to a reduced risk to businesses.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Asia-Pacific Clinical Trial Supplies Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/asia-pacific-clinical-trial-supplies-market
Asia-Pacific Clinical Trial Supplies Market Overview
**Segments**
- Based on product and services, the Asia-Pacific clinical trial supplies market can be segmented into manufacturing, packaging, labeling, and distribution services. The demand for these services is growing due to the increase in clinical trial activities in the region as pharmaceutical and biotechnology companies look to expand their presence in emerging markets. Additionally, the growing emphasis on quality and compliance in clinical trials is driving the need for specialized services to ensure that supplies meet regulatory requirements.
- On the basis of phase, the market can be segmented into Phase I, Phase II, Phase III, and Phase IV clinical trials. Each phase has different requirements in terms of supplies, with Phase I focusing on initial safety and tolerance testing, while Phase IV involves post-marketing surveillance. The demand for supplies varies across these phases, with Phase III trials typically requiring the largest volume of supplies as they involve a larger number of patients.
- By therapeutic area, the Asia-Pacific clinical trial supplies market can be segmented into oncology, cardiovascular diseases, infectious diseases, central nervous system disorders, and others. The increasing prevalence of chronic diseases in the region is driving the demand for clinical trials in these therapeutic areas, leading to a growing need for supplies to support research in oncology, cardiovascular diseases, and other key areas.
**Market Players**
- Some of the key players in the Asia-Pacific clinical trial supplies market include Thermo Fisher Scientific Inc., Catalent, Inc., Parexel International Corporation, PCI Pharma Services, Charles River Laboratories, Inc., KLIFO A/S, Almac Group, AptarGroup, Inc., Sharp Clinical Services, Inc., and Thermo Scientific. These companies offer a wide range of products and services to support clinical trials, including packaging, labeling, and distribution services. They have a strong presence in the region and are focused on expanding their offerings to meet the growing demand for clinical trial supplies.
- Other players in the market include Movianto, Bellwyck Pharma Services, Myoderm, and Bilcare Limited. These companies provide specialized services in clinical trial supplies, such as cold chain logistics and direct-to-patient shipping, to support the complex requirements of modern clinical trials. They play a crucial role in ensuring the efficient and timely delivery of supplies to clinical trial sites, helping to streamline the research process and accelerate the development of new therapies in the region.
The Asia-Pacific clinical trial supplies market is witnessing significant growth driven by several key factors. One of the primary drivers is the increasing focus on expanding clinical trial activities in the region by pharmaceutical and biotechnology companies. This trend is fueled by the growing patient population, rising healthcare expenditure, and advancements in healthcare infrastructure. As companies seek to tap into the diverse patient pool in Asia-Pacific, the demand for clinical trial supplies is expected to rise steadily.
Moreover, the emphasis on quality and compliance in clinical trials is also boosting the market as companies look to ensure that their supplies meet regulatory standards. This has led to a growing need for specialized services such as manufacturing, packaging, labeling, and distribution services to support the conduct of clinical trials effectively and efficiently. As the regulatory landscape continues to evolve, companies are increasingly turning to service providers that can offer expertise in navigating complex regulatory requirements.
In terms of market players, the Asia-Pacific clinical trial supplies market is characterized by a competitive landscape with several key players vying for market share. Companies such as Thermo Fisher Scientific Inc., Catalent, Inc., and Parexel International Corporation are at the forefront of offering a diverse range of products and services to support clinical trials in the region. These players are investing in research and development to enhance their offerings and cater to the evolving needs of the market.
Furthermore, the market also includes a number of other players such as Movianto, Bellwyck Pharma Services, and Myoderm, who provide specialized services in areas like cold chain logistics and direct-to-patient shipping. These companies play a crucial role in ensuring the efficient and timely delivery of supplies to clinical trial sites, thereby facilitating the smooth conduct of research activities. With the increasing complexity of clinical trials and the need for tailored solutions, these specialized service providers are expected to play an increasingly important role in the Asia-Pacific market.
Overall, the Asia-Pacific clinical trial supplies market is poised for continued growth driven by factors such as increasing clinical trial activities, regulatory requirements, and the presence of key market players offering a wide range of products and services. As the region continues to attract attention from global pharmaceutical and biotechnology companies, the demand for clinical trial supplies is expected to rise, creating opportunities for both established players and emerging service providers to expand their presence and capitalize on this growing market.The Asia-Pacific clinical trial supplies market is a dynamic and rapidly evolving sector with several key trends shaping its growth trajectory. One significant trend driving market expansion is the increasing focus on personalized medicine and targeted therapies. As advancements in genomics and molecular biology continue to accelerate, there is a growing emphasis on developing treatments tailored to individual patients' genetic profiles. This trend is driving a surge in clinical trials focusing on precision medicine, thereby increasing the demand for specialized supplies and services to support this research.
Another key trend in the market is the rising adoption of innovative technologies such as artificial intelligence (AI) and big data analytics in clinical trial design and execution. These technologies offer the potential to streamline trial processes, enhance data collection and analysis, and improve patient recruitment and retention rates. As companies in the Asia-Pacific region look to leverage these technologies to enhance the efficiency and effectiveness of their clinical trials, the demand for advanced supplies and services that can integrate with these technologies is expected to grow.
Furthermore, the increasing focus on patient-centricity in clinical trials is reshaping the landscape of trial supplies and services. Patient-centric approaches emphasize the importance of incorporating patient perspectives and preferences into trial design and implementation to improve patient engagement, recruitment, and retention. This shift towards patient-centric trials is driving the need for supplies and services that can support decentralized and virtual trial models, as well as direct-to-patient shipping solutions to enhance patient convenience and participation rates.
Additionally, the Asia-Pacific region's growing significance as a hub for clinical research and development is attracting attention from global pharmaceutical and biotechnology companies seeking to capitalize on the diverse patient populations and regulatory environments in countries such as China, Japan, and South Korea. This influx of multinational companies is driving increased investment in clinical trials in the region, leading to a corresponding rise in demand for high-quality supplies and services to support these trials.
Overall, the Asia-Pacific clinical trial supplies market is poised for sustained growth driven by trends such as the focus on personalized medicine, adoption of innovative technologies, emphasis on patient-centricity, and the region's emergence as a key hub for clinical research. Companies operating in this market will need to adapt to these trends by offering specialized supplies and services that cater to the evolving needs of the industry, thereby positioning themselves for success in this dynamic and competitive landscape.
The Asia-Pacific Clinical Trial Supplies Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/asia-pacific-clinical-trial-supplies-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Answers That the Report Acknowledges:
- Market size and growth rate during forecast period
- Key factors driving the Asia-Pacific Clinical Trial Supplies Market
- Key market trends cracking up the growth of the Asia-Pacific Clinical Trial Supplies Market.
- Challenges to market growth
- Key vendors of Asia-Pacific Clinical Trial Supplies Market
- Opportunities and threats faces by the existing vendors in Global Asia-Pacific Clinical Trial Supplies Market
- Trending factors influencing the market in the geographical regions
- Strategic initiatives focusing the leading vendors
- PEST analysis of the market in the five major regions
Browse More Reports:
Global Offshore Mooring Systems Market
Global Industrial Backhoe Loader Market
Global Hybrid and Community Cloud as a Service Market
North America Stand-Up Paddleboard Market
Global Eggshell Membrane Powder Market
Global Aluminium Composite Panels Market
Global Bulk Chemical Drums Market
Asia-Pacific Pharmaceuticals Packaging Testing Equipment Market
Global Composite Materials Market
Global Semi-Finished Pastry Ingredients Market
Global Fragrance Packaging Market
Global Electrical Discharge Machine (EDM) Market
Global Bio-Based Polypropylene (PP) Market
Global Flat Back Tape Market
Global 3D Laser Scanner Market
North America Freight Transportation Management Market
Asia-Pacific API Intermediates Market
North America Defibrillators Market
Asia-Pacific Smoked Cheese Market
Europe Soft Tissue Repair Market
Global Crambe Abyssinica Seed Oil Market
Europe Anthrax Treatment Market
Global Dog Food Extrusion Market
Global Waterproof Coatings and Membranes Market
Australia and New Zealand Non-Stick Cookware Market
Global Cerebral Palsy Market
Global Snow Pushers Market
Global Electro Pneumatic Train Brakes Market
Global Loyalty Management Market
Global Connected Living Room Market
Global Specialty Oilfield Chemicals Market
Global Botanical Ingredients for Neutraceutical Market
Global Residential Portable Air Purifier Market
North America Chinese Hamster Ovary (CHO) Cells Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
Asia-Pacific Clinical Trial Supplies Market, Asia-Pacific Clinical Trial Supplies Market Trends, Asia-Pacific Clinical Trial Supplies Market Growth, Asia-Pacific Clinical Trial Supplies Market Demand, Asia-Pacific Clinical Trial Supplies Market Size, Asia-Pacific Clinical Trial Supplies Market Scope, Asia-Pacific Clinical Trial Supplies Market Insights, Asia-Pacific Clinical Trial Supplies Market Analysis